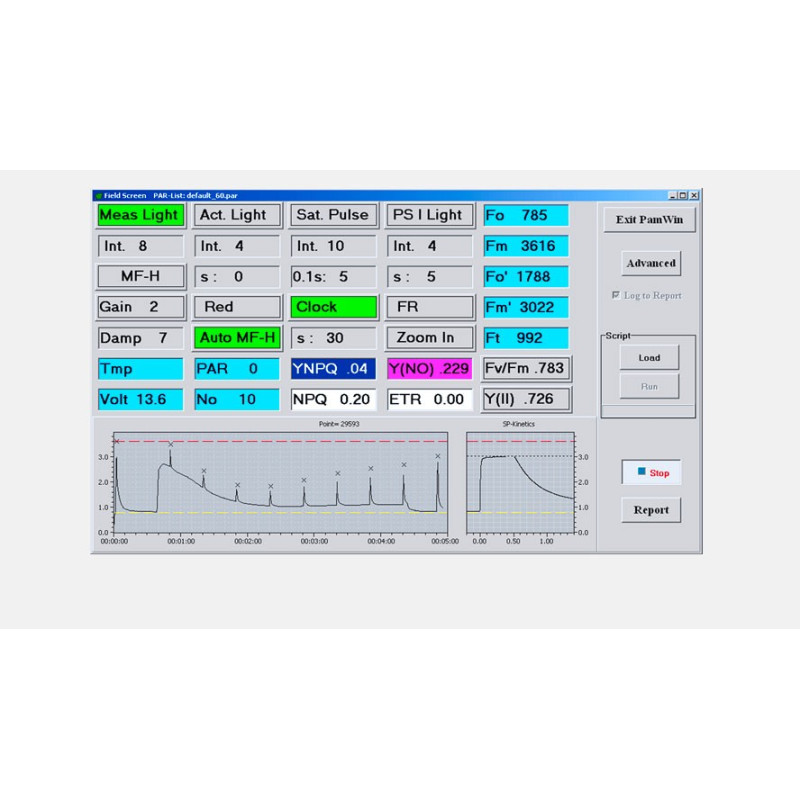
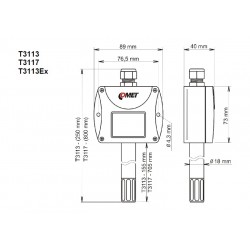
LIGHT SENSORS & ACCESSORIES
2060-A Fiberoptics Holder for Surfaces WALZ
LIGHT SENSORS & ACCESSORIES
2060-M Mini-Quantum/Temp.-Sensor WALZ
LIGHT SENSORS & ACCESSORIES
MKS-2500 Magnetic Stirrer with Fiberoptics Holder WALZ
LIGHT SENSORS & ACCESSORIES