Cart
0
Product
Products
(empty)
No products
Free shipping!
Shipping
0,00 €
Total
Prices are tax excluded
Product successfully added to your shopping cart
Quantity
Total
There are 0 items in your cart. There is 1 item in your cart.
Total products
(tax excl.)
Total shipping (tax excl.)
Free shipping!
Total
(tax excl.)
Catalog
Membranes for Fuel Cells
-
PRODUCTS
- WEATHER STATIONS
- WATER QUALITY MONITORING
- TRANSMITTERS AND TRANSDUCERS
- TRAINING & EDUCATION SYSTEMS
- SPECTROMETERS & SPECTRO-RADIOMETERS
- SENSORS & PROBES
- POWER & ENERGY
- MEASUREMENT AND CALIBRATION INSTRUMENTS
- LIGHT & LASER SOURCES
-
LABORATORY, PHARMACY & MEDICINE EQUIPMENT
- Water Quality
- Vet
- VARIOUS
- VACUUM SYSTEMS
- Ultrasonic Baths
- Stirrers
- SPECTRO-PHOTOMETERS
- Showers
- Sensores y Sondas
- Rotary Evaporator
- Refractometers
- Pumps
- PRECISION BALANCES
- Plant Growth Chambers
- PARTICLE ANALYZERS
- OVENS
- ORBITAL SHAKERS
- Mills
- MILK ANALYZERS
- Microtome
- Microscopes
- Microplate Readers
- MEDICAL ANALYZERS
- Laboratory Reactors
- INFLAMMATION ANALYZERS
- INCUBATORS
- HUMAN TEMPERATURE
- Hotplates
- GAS BURNERS
- Gas Analyzers & Detectors
- FAT ANALYZER
- Evaporators
- Distillers
- Dissolved Oxygen
- Density Meters
- Cultivators
- COLORIMETERS/GLOSS METERS
- COLD STORAGE ROOMS
- Chromatograph
- Chillers, Coolers, Refrigerators
- Chemical Analyzers
- CENTRIFUGERS
- CABINETS AND HOODS
- Body Scanners
- BATHS AND CIRCULATORS
- AUTOCLAVES
- ANALYSERS
- Anaerobic Workstations/Chambers
- AIR QUALITY
- Air Purify
- Instruments for Geophysics and Seismology
- INDUSTRY & PROCESS CONTROL
-
HAND/PORTABLE METERS
- WOOD MOISTURE
- Weather Station
- WATER QUALITY
- Water Flow
- VIDEOSCOPE
- VIBRATION ANALYZERS
- Veterinary
- ULTRAVIOLET
- Thickness Measurement
- THERMAL IMAGING
- TEMPERATURE & HUMIDITY
- TEMPERATURE
- TDS
- Spectrum Analyzers
- Spectrocolorimeters
- SPECTRO RADIOMETERS
- SPECTRO PHOTOMETERS
- Sound Meter
- SOUND LEVEL METERS
- SOLAR RADIATION
- Soil Moisture
- SOIL COMPACTATION
- SOIL CALCIMETER
- Salinity
- Relative Humidity
- Refractometers
- RADIATION (Gamma, Beta and X)
- Quantum Light 0-4000 µmol
- PRESSURE & TEMPERATURE
- Pressure
- Power Analyzers
- PHOTOSYNTHETIC LIGHT
- pH
- Particles in Air
- OXIGEN
- ORP
- OIL ANALYZERS
- MULTIFUNCTION
- LUXOMETROS
- Leakage Meters & Detectors
- ION MEASUREMENTS
- INFRARED Temp. RADIOMETER
- Humidity Sawdust/Hay/Pellets
- HUMIDITY OF CONCRETE
- GLOSS
- GAS ANALYZERS
- FORMALDEHYDE
- Food
- Fluorometers
- Distance rangefinder
- DISSOLVED OXYGEN
- Density
- CONDUCTIVITY METERS
- CONDUCTIVITY
- Colorimeters
- CO2 (Carbon Dioxide)
- CO (Monóxido de Carbono)
- CHLOROPHYLL CONCENTRATION
- Cement Moisture
- ANEMOMETER
- AIR QUALITY
- ACCESORIES
- FUEL CELL HARDWARE
- FLOW METERS
- ENVIRONMENTAL PRODUCTS
- Environmental Hygiene
- ELECTROCHEMISTRY, MATERIAL SCIENCE & IMPEDANCE
- ELECTRICITY NETWORK ANALYZERS
- DATA LOGGERS
- CLOROPHYL PHOTOSYNTHESIS
- CATALOGS
-
Browse by Measurement
- Wood Moisture
- Wind Direction
- Water Quality
- Water Level
- WATER FLOW
- VOC
- Viscosity
- Vibration Analyzers
- Vet
- V & mV
- Ultraviolet Light
- Turbidity
- Time
- Thickness
- Temperature
- Telemetry
- TDS
- Strain Gauges
- Stomatal Conduct.
- Sterilization
- States (ON/OFF)
- Spectroradiometry
- Spectrophotometry
- Spectrometry
- Solar Radiation
- Soil Water Potential
- Soil Moisture
- Sodium
- Snow Depth
- Seismology
- Sap/Sap Flow
- Sap Flow
- Salinity/Conductivity
- RELAY OUTPUTS
- Relative Humidity
- Rain Gauge
- Radon
- Radiation
- Quantum Light
- Pulses
- Pressure
- Potassium
- Pipes Inspection
- Photosynthesis
- Pharmacy & Medicine
- pH
- Peristaltic Pumps
- Penetrometers
- Particle Meters
- Particle Counters
- PAR Light
- Ozone (O3)
- Oxígeno Disuelto DO
- ORP - REDOX
- Occupation and Time
- O2 Oxygen
- Noise
- Nitrogen Generators
- Nitrate
- Network Analyzers
- Movement & GPS
- Meteorology
- Load Cells
- Light & Laser Sources
- Level
- Leaf Wetness
- Leaf area index
- LCR
- KW & KW/H
- Ions
- Impedance
- Idc (DC Current)
- Iac (AC Current)
- Haze/Fog
- GEOTECHNICS
- Geiger counters
- GAS (ANALYZERS)
- Fuel Cells
- Fluorescence
- Flavor/Taste/Acidity
- Exterior humidity
- Events
- Evapotranspiration
- Energy and Power
- Electrochemistry
- Distance
- Differential Pressure
- Diesel Level
- Dew Point
- Destiladores
- Density
- Dendrometers
- Cryoscopes
- Cracks in Buildings
- Conductivity in Water
- Conductivity in Soil
- COMPRESSED AIR
- Color / Colorimeter
- CO2 Carbon Dioxide
- CO Carbon Monoxide
- Chlorophyll
- Calibrators
- Calcium
- Autoclaves
- Atmospheric Pressure
- Ammonia
- Airflow
- Air Speed
- Air quality
- 4-20mA
- Highlighted
-
Brands
- WonATech
- Walz
- Veris Industries
- Vegetronix
- UPWARD INNOVATIONS
- Tempmate
- Tanel
- TandD
- StellarNet .Inc
- Spectrum Technologie
- SolGeo
- SCRIBNER ASSOCIATES
- Scientech Tech.
- RST Instruments
- RIKA SENSORS
- RADONOVA
- Quarta-Rad
- PSI (Photon Systems)
- Pronamic
- Particles Plus
- Onset
- Nvis Technologies
- NorECs
- NexCeris - NextCell
- Nesa
- MRC Lab. Equipment
- Meter Environment
- MEATROL
- Magnelab
- MAE srl
- Konted
- KandH
- Judd Communications
- IMKO
- iButton
- Hubei Fangyuan
- HORIBA/Laqua
- HONGYUV
- Grant Instruments
- Gentos
- Fuehler Systeme
- FRIGGA
- EMS Brno
- EME Systems
- ELECTROCHEM INC.
- Eko Instruments
- Ecomatik
- Delta-T
- Delta Ohm (Senseca)
- Decagon
- Davis
- Comet System
- Chao Sensor
- Capetti Elettronica
- Bosean
- Blue Maestro
- Autoedu
- Atago
- Aquas Inc.
- Aquaread
- Apogee Instruments
- AO-Electronics
- Algodue
- AIRY TECHNOLOGY
- Acksen
- 3nh
- Spectral Evolution
-
Applications
- Water
- Veterinary
- TRAINING / EDUCATIONAL
- SPECTROSCOPY & SPECTROPHOTOMETRY
- SCIENCE AND RESEARCH
- MEASUREMENT AND CALIBRATION INSTRUMENTS
- LABORATORY, PHARMACY, MEDICINE AND HEALTH
- INDUSTRY
- Geotechnics /Seismology
- ENVIRONMENTAL SCIENCES
- ENVIRONMENTAL HYGIENE
- Cold Chain - Transport
- Building Monitoring
- AUTOMOTIVE
- AUTOCLAVES
fuentes RSS
No RSS feed added

Membranes for Fuel Cells
For a PEM fuel cell to operate correctly, a Proton Exchange Membrane is needed that will carry the hydrogen ions, proton, from the anode to the cathode without passing the electrons that were removed from the hydrogen atoms.
These Polymer Membranes that conduct the protons through the membrane but are reasonably impermeab...
Membranes for Fuel Cells
For a PEM fuel cell to operate correctly, a Proton Exchange Membrane is needed that will carry the hydrogen ions, proton, from the anode to the cathode without passing the electrons that were removed from the hydrogen atoms.
These Polymer Membranes that conduct the protons through the membrane but are reasonably impermeable to the gases, serve as solid electrolytes (vs. liquid electrolyte) for variety of electrochemical applications, and are commonly known as Proton Exchange Membrane and/or Polymer Electrolyte Membranes (PEM). These membranes have been identified as one of the key components for various consumer related applications for fuel cells, e.g. automobiles, back-up power, portable power etc. Due to its application for many consumer markets, the technology keeps on evolving to make these membranes suitable for longer duration, and even high temperature operations.
For PEM fuel cells and electrolyzer applications, a polymer electrolyte membrane is sandwiched between an anode electrode and a cathode electrode. During electrochemical reaction, oxidation reaction at the anode generates protons and electrons; reduction reaction at the cathode combines protons and electrons with oxidants to generate water. To complete the electrochemical reaction, the proton exchange membrane plays a critical role that conducts protons from anode to cathode through the membrane. The proton exchange membrane also performs as a separator for separating anode and cathode reactants in fuel cells and electrolyzers.
Main Types of Fuel Cell Ion Exchange Membranes:
- Cation Exchange Membrane (CEM)
- Anion Exchange Membrane (AEM)
- Bipolar Membranes
- Chlor-alkali Production Membranes
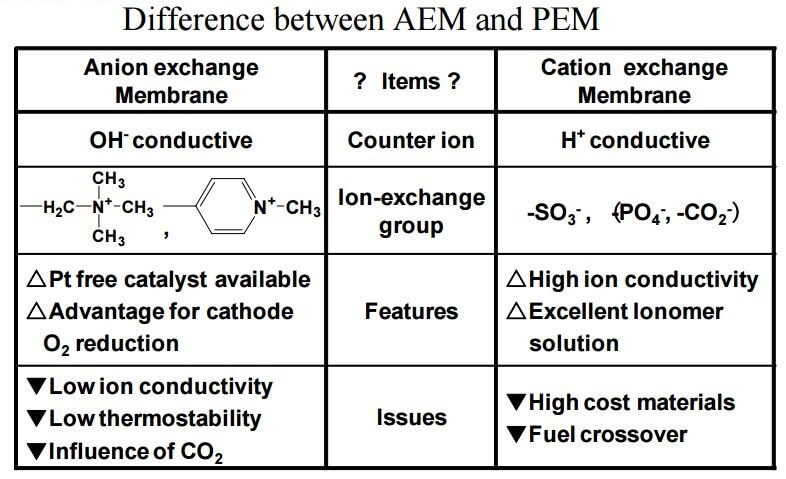
Whats are the Differences Between Cation and Anion Exchange Membranes?:
- Cation Exchange Membranes (CEM) are usually comprised of a fluorinated polymer with sulfonic acid sites and have excellent ionic conductivity and thermal/chemical durability. Currently manufactured - Anion Exchange Membranes (AEMs) can utilize various alkaline stable polymeric materials as the host material and come with various functional sites that conducts OH- or any other anionic species.
- Thermal/chemical durability of AEMs are in general lower compared to its CEM counterparts.
Showing 1 - 2 of 2 items
-
Xion ™ PEM-Nafion-1000-05 Membrane - AO-72600082
The AO-72600082 Xion™ Composite PEM-Nafion-1000-05 is a 5 micrometers thick composite proton exchange membrane that uses the Nafion resin with an equivalent weight of 1000. It is stable under acidic and neutral conditions. This membrane can be used in fuel cells, electrolyzers, electrodialysis, redox flow batteries, electrochemical compressors, sensors,...
Showing 1 - 2 of 2 items




